The combination of six immunotherapies is a type of immunotherapy for cancer, in which six types of immune cells present in the blood are extracted, activated, and expanded to enhance their ability to effectively attack cancer cells. These activated immune cells are then reintroduced into the body, boosting its ability to fight against cancer.
The immune cells, which have been primed with peptide information specific to the targeted cancer, target and attack both cancer cells and cancer stem cells. This therapy is applicable for the treatment and prevention of almost all types of cancer, as well as for preventing cancer metastasis and recurrence after surgery.
The treatment is simple, involving only blood collection and intravenous infusion. It is a cell-based therapy using the patient's own cells, so it has minimal side effects and imposes minimal burden on the body.


The immune cells, which have been expanded to approximately 300 times their original quantity, attack cancer cells.
In the 6 Combination Immunotherapy, immune cells are extracted from the blood and successfully activated and proliferated to enhance their ability to attack cancer. Actual measurement of the immune cell count has confirmed that they increased by approximately 300 times after a 20-day cultivation period.
Introduction of treatment records
We would like to introduce the treatment results of a patient who completed six rounds (one course) of treatment. After the treatment, the size of the tumor was assessed by the physician, and the progression inhibition rate was approximately 76% (109 out of 144 individuals).
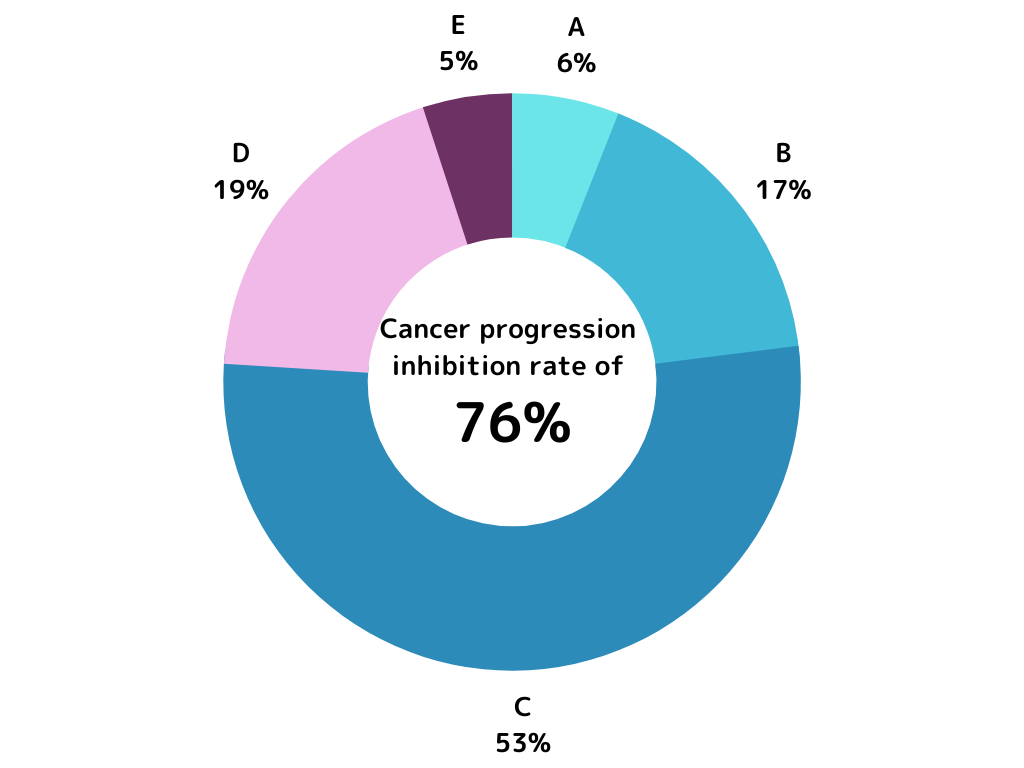
A assessment to C assessment: Progression has been inhibited as evaluated.
Number of participants: 144
Survey period: June 2020 to December 2022
A assessment: Significant reduction, substantial decrease in tumor size.
B assessment: Reduction, noticeable decrease in tumor size.
C assessment: No change, no significant change in tumor size.
D assessment: Slightly increased, slight enlargement of the tumor observed.
E assessment: Increased, noticeable enlargement of the tumor observed.
Treatment effectiveness rate: Evaluated based on the RECIST guidelines, an internationally recognized standard for treatment response assessment.

Treatment through blood collection and intravenous infusion
When performing the treatment, the first step is to collect blood from the patient. The blood is then sent to the cell processing center, where six types of immune cells are carefully extracted. The extracted immune cells are further multiplied and activated over a period of approximately 20 days, and then reintroduced into the patient's body through intravenous infusion.

Side effects and precautions
The frequency of side effects is not high, and there are no reports of severe adverse reactions. However, transient fever, redness, rash, and itching at the injection site may occur. Concurrent use with immune checkpoint inhibitors may increase the risk of serious side effects, so it should be carefully considered.
Since a cultivation period is required, it takes about three weeks from blood collection to administration. If the cells do not meet the required standards due to contamination (such as bacterial contamination) during the cultivation process, or if there are difficulties in transporting the cultured cells due to the patient's condition or natural disasters, it may be necessary to repeat the blood collection and cultivation.
The treatment response may vary depending on the individual cases, and in some cases with advanced disease, no treatment effect may be observed.

Treatment prices
| 1 time | ¥550,000 (¥605,000 including tax) |
| 6 times | ¥3,200,000 (¥3,520,000 including tax) |
